Pipeline
OTT166 is a novel small molecule selective integrin inhibitor that OcuTerra has purpose engineered to have the required physiochemical characteristics to be able to reach the retina from eye drop application.

Pipeline
OTT166 is a novel small molecule selective integrin inhibitor that OcuTerra has purpose engineered to have the required physiochemical characteristics to be able to reach the retina from eye drop application.
OTT166, has been studied in two separate multi-center, randomized, Phase 1b trials, in retinal disease patients and shown safety, tolerability and evidence of biological activity.
OcuTerra is currently studying the safety, efficacy and optimal dosing regimen of OTT166 through the Phase 2 DR:EAM (Diabetic Retinopathy: Early Active Management) study in patients with moderately-severe to severe non-proliferative and mild proliferative diabetic retinopathy.
Optical Coherence Tomography (OCT) images from 3 patients with Diabetic Macular Edema (DME) demonstrating Central Retinal Thickness (CRT) improvement and a clear biologic effect of topical OTT-166
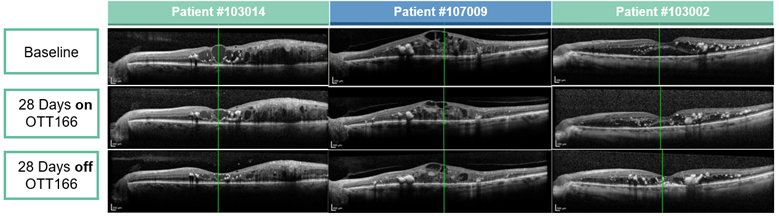
| Central Retinal Thickness (CRT)µm | ||||||
|---|---|---|---|---|---|---|
| Patient | #103014 | #107009 | #103002 | |||
| Baseline | 530 | 766 | 404.5 | |||
| Day 28 | 327.5 | -202.5 (-38%) | 570 | -196 (-25%) | 288.5 | -116 (-29%) |
| Day 56 | 267 | -263 (-50%) | 577 | -189 (-25%) | 243.5 | -161 (-40%) |
| Central Retinal Thickness (CRT)µm | ||
|---|---|---|
| Patient | #103014 | |
| Baseline | 530 | |
| Day 28 | 327.5 | -202.5 (-38%) |
| Day 267 | 267 | -263 (-50%) |
| Patient | #107009 | |
| Baseline | 766 | |
| Day 28 | 570 | -196 (-25%) |
| Day 267 | 577 | -189 (-25%) |
| Patient | #103002 | |
| Baseline | 404.5 | |
| Day 28 | 288.5 | -116 (-29%) |
| Day 267 | 243.5 | -161 (-40%) |
Learn more about