For Physicians
DR:EAM: a study of OTT166, a novel investigational candidate for the treatment of diabetic retinopathy

For Physicians
DR:EAM: a study of OTT166, a novel investigational candidate for the treatment of diabetic retinopathy
The DR:EAM clinical trial is a randomized, double-masked, vehicle-controlled, Phase 2 study that will evaluate the safety and efficacy of OTT166 ophthalmic solution in certain adults with diabetic retinopathy, which is being conducted in the United States and Puerto Rico. For more information, visit clinicaltrials.gov
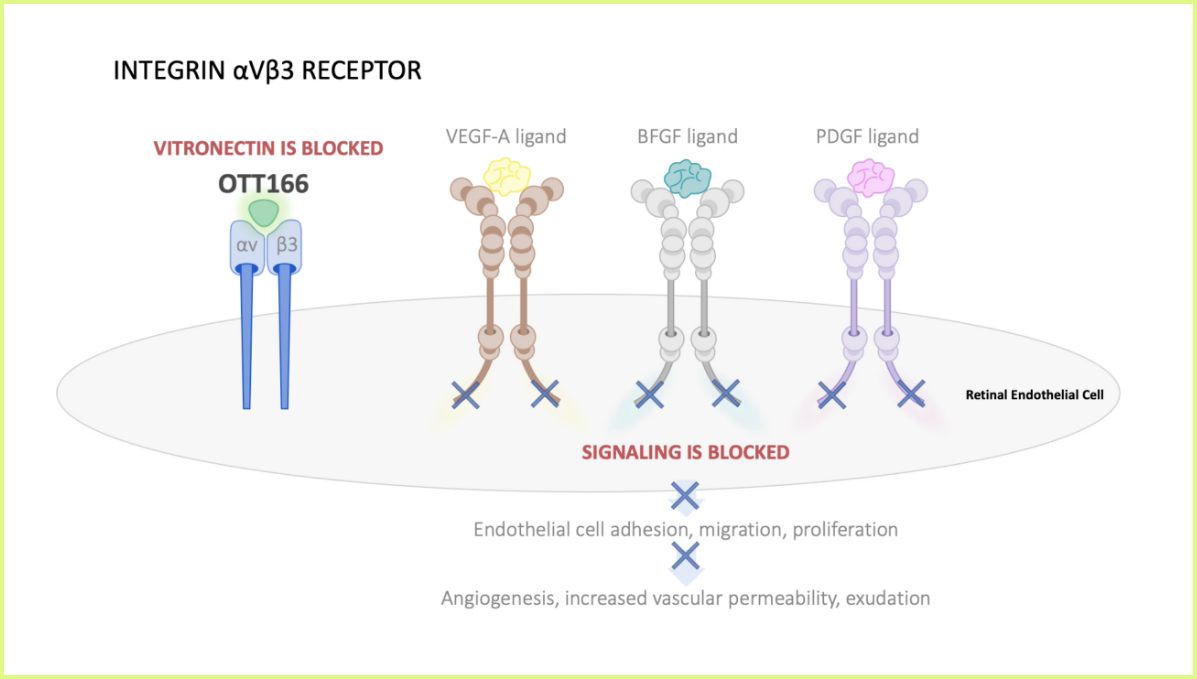
OTT166 is designed as an integrin inhibitor with anti-VEGF and other growth factor modulating effects with the potential to modulate multiple drivers of DR. As an investigational therapeutic delivered via eye drop, OTT166 has the potential to provide a non-invasive option for patients with DR.
The primary efficacy endpoint of the DR:EAM clinical trial is the percentage of patients that have a 2-step improvement in the Diabetic Retinopathy Severity Scale (DRSS). Additional endpoints of the clinical trial include measuring the prevention of progression to vision-threatening complications, amount of delayed time to intravitreal injection and/or laser treatment, and exploratory imaging endpoints.